FDA Cybersecurity Guidance: Elevate Your SSDLC & SPDF
Why the 2025 FDA Cybersecurity Update Matters
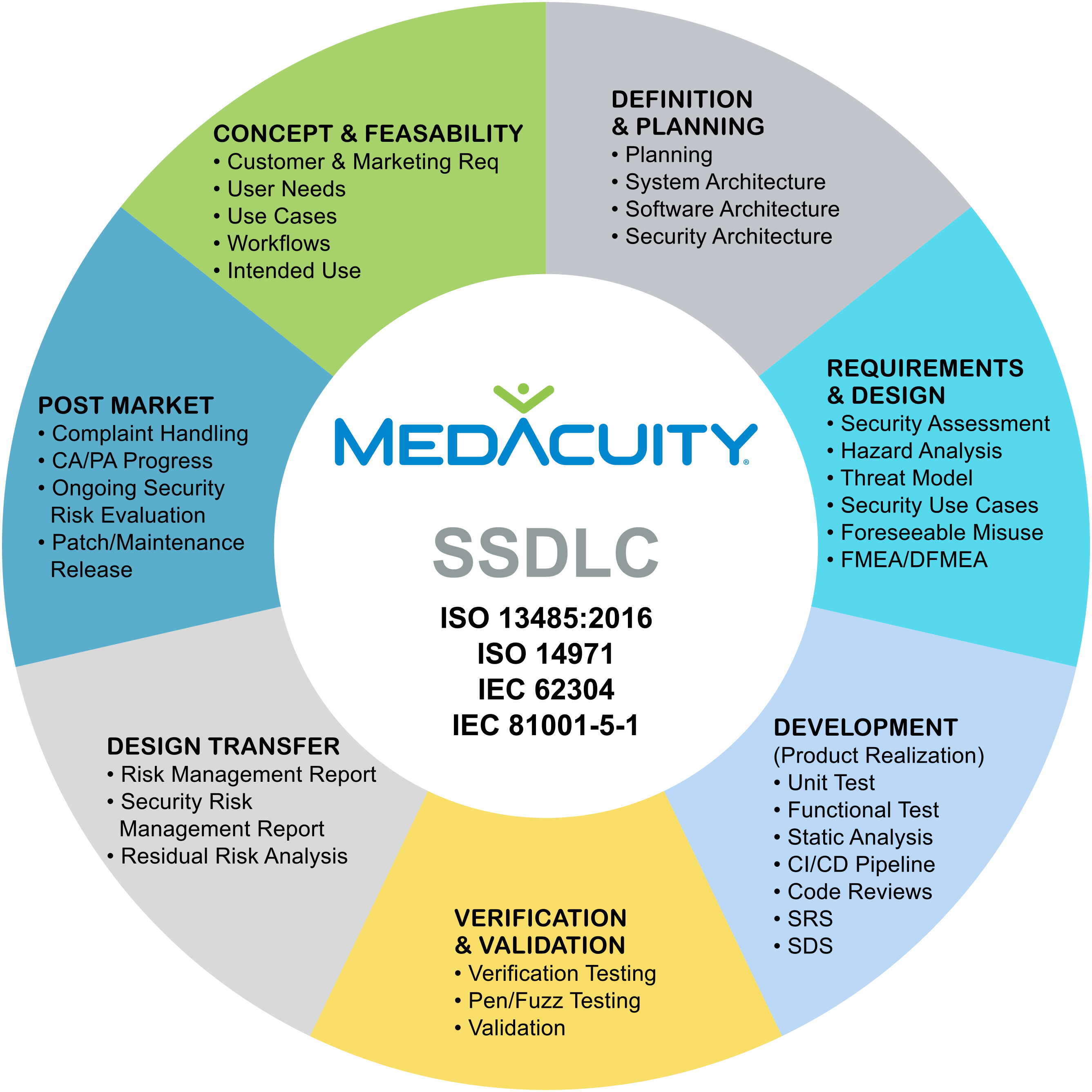
FDA Cybersecurity Guidance SSDLC SPDF: What You Need to Know
On June 27, 2025, the FDA released its latest cybersecurity guidance, making it clear: implementing a Secure Software Development Lifecycle (SSDLC) within a Secure Product Development Framework (SPDF) is now a regulatory expectation, not just a recommendation.
If you’re developing or maintaining software for connected medical devices, “secure by design” is no longer optional, it’s essential.
This guidance marks a pivotal shift, placing greater accountability on manufacturers to proactively embed security into every phase of the development lifecycle, from concept to post market support.
SSDLC + SPDF = Compliance + Confidence
A mature SSDLC and SPDF not only align with FDA expectations — they also:
✅ Minimize vulnerabilities early
✅ Lower remediation costs
✅ Accelerate innovation without compromise
✅ Strengthen your product’s marketability and safety profile
MedAcuity: Your Partner in Secure, FDA-Ready Software Development
At MedAcuity, we help MedTech leaders design, build, and maintain cyber resilient medical software that meets evolving FDA requirements, without slowing innovation.
Our FDA Cybersecurity Readiness Services Include:
- Implementing SSDLC within a compliant SPDF
- Integrating DevSecOps into CI/CD pipelines
- Risk-based cybersecurity assessments
- Conducting SPDF/SSDLC gap analysis against FDA expectations
- Supporting SBOM creation, threat modeling, and secure architecture
With deep expertise in regulated environments, we help you reduce risk, improve development efficiency, and stay audit ready. Learn more about how MedAcuity’s cybersecurity and compliance services can help you implement SSDLC and SPDF effectively.
Secure Software by Design. Ready for What’s Next.
Whether you’re preparing a premarket submission or addressing post market cybersecurity obligations, our team helps you build FDA aligned processes that are scalable, testable, and audit ready.
Don’t wait until the audit. Prepare now.
📅 Schedule a consultation with our team to assess your current software security posture and begin your journey toward FDA cybersecurity alignment.
Expertise
Get to know our capabilities better. Check out some of our Insights articles and recent projects.
INSIGHTS ARTICLES
Healthcare Cybersecurity Challenges: Securing Medical Devices & Data Protection
Effective Strategies for OEMs
Project Snapshots

Clear Requirements: A Blueprint for Success
How clear & well-defined requirements shape software development success
White Paper

Selecting a Cybersecurity Partner for Medical Device Development
Start building more secure, reliable medical technologies today
