Time Is Ticking: Prepare Now for QMSR Compliance by the 2026 Deadline
FDA Final Rule Aligns QMSR with ISO 13485:2016
On February 2, 2024, the FDA issued the Final Rule amending 21 CFR Part 820, officially aligning its Quality Management System Regulation (QMSR) with ISO 13485:2016, the internationally harmonized standard for medical device quality systems. This long anticipated move modernizes FDA requirements and streamlines global compliance by syncing with standards already recognized by the European Union (EU) and other key markets.
The Final Rule provides a two year transition period, meaning enforcement begins February 2, 2026. That may sound distant, but in the regulatory world, it’s just around the corner. Now is the time to assess and align your Quality Management System (QMS) to ensure compliance before the clock runs out.
What Does the QMSR Final Rule Mean for You?
The core of the Final Rule involves amending 21 CFR Part 820 by incorporating ISO 13485 by reference. Rather than maintaining a separate and sometimes conflicting set of FDA specific quality system requirements, the agency is now fully harmonizing with the globally accepted ISO framework.
Key Takeaways:
- ISO 13485:2016 now forms the backbone of FDA QMS requirements.
- Harmonization reduces regulatory redundancy and eases global market access.
- Legacy QMS processes must be reviewed and updated to reflect the ISO based approach.
Why Early QMSR Preparation Is Critical
With the enforcement date rapidly approaching, organizations that delay QMS remediation may face:
- Regulatory delays
- Product rework and recalls
- Increased audit findings
- Limited market access
Don’t Stop at QMSR – Update for Cybersecurity Compliance Too
As you work to align your QMS with the QMSR Final Rule, it’s also an ideal opportunity to integrate the latest FDA cybersecurity guidance, issued June 27, 2025. This updated guidance emphasizes the need for a Secure Product Development Framework (SPDF) and a Secure Software Development Lifecycle (SSDLC), both crucial for regulatory compliance and market trust.
With the increasing focus on software intensive medical devices, cybersecurity is no longer optional. Integrating SSDLC and SPDF principles into your QMS will not only align you with FDA expectations but also improve your security posture and product resilience.
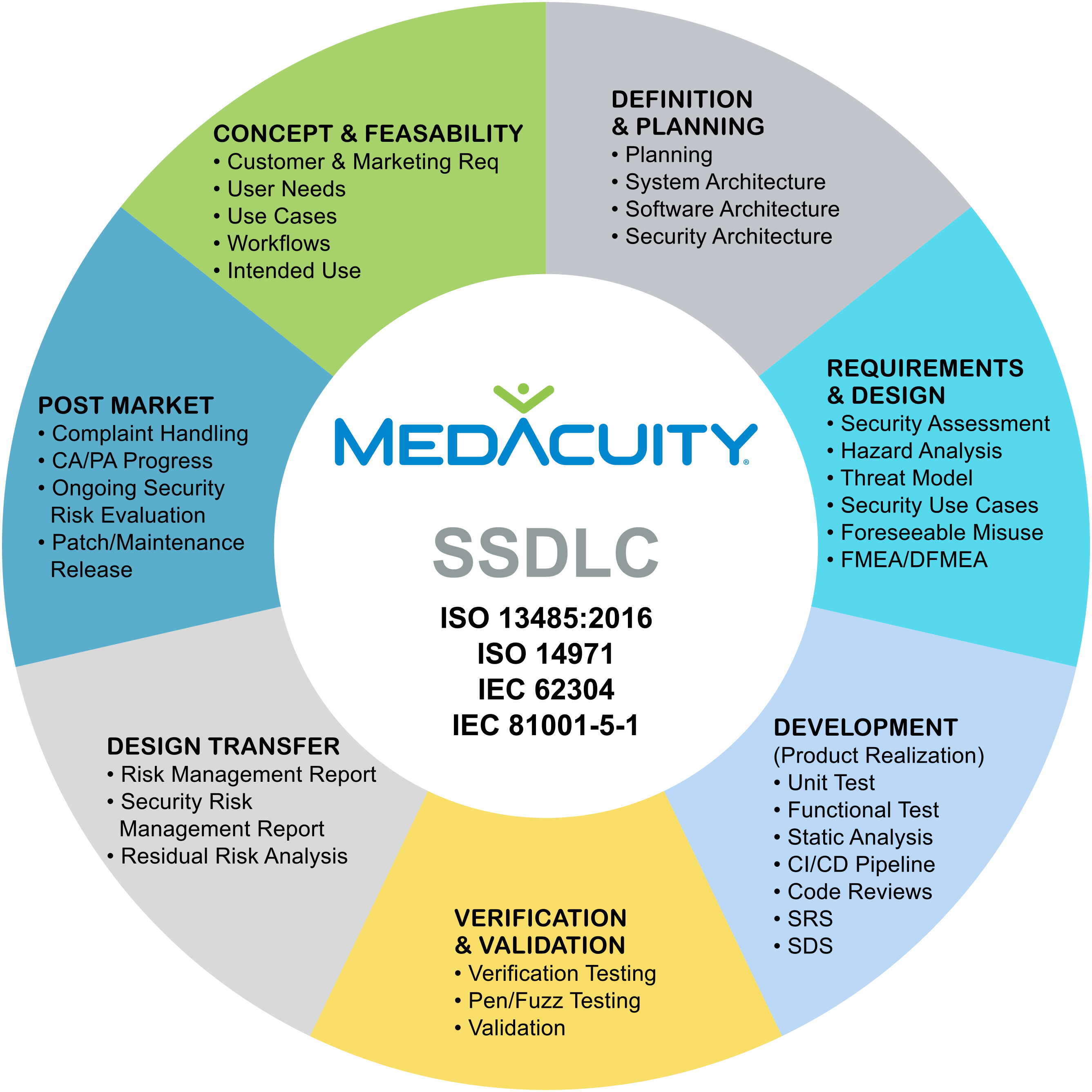
How MedAcuity Can Support Your Compliance Journey
MedAcuity offers deep expertise in regulatory compliance, QMS remediation, and cybersecurity integration.
Our team can help you:
✅ Perform a QMS gap analysis against ISO 13485 and the QMSR Final Rule
✅ Update your QMS to align with the latest cybersecurity guidance
✅ Integrate SSDLC and SPDF into your development and quality systems
Let us help you navigate these critical updates before the time pressure becomes a crisis.
Contact MedAcuity today to schedule your QMS review and ensure your organization is on track for compliance by February 2, 2026.
Expertise
Get to know our capabilities better. Check out some of our Insights articles and recent projects.
INSIGHTS ARTICLES
Healthcare Cybersecurity Challenges: Securing Medical Devices & Data Protection
Effective Strategies for OEMs
Project Snapshots

Clear Requirements: A Blueprint for Success
How clear & well-defined requirements shape software development success
White Paper

Selecting a Cybersecurity Partner for Medical Device Development
Start building more secure, reliable medical technologies today

